Galvanic batteries, also known as electrochemical cells, are essential components in modern technology, powering everything from small electronics to electric vehicles. In this blog, we will explore the fundamentals of galvanic batteries, their components, how they work, and their diverse applications.
What is a Galvanic Battery?
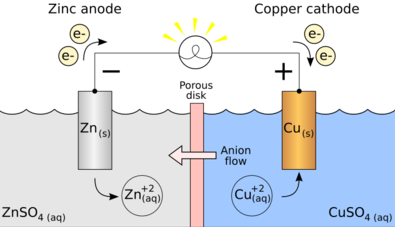
A galvanic battery is a device that converts chemical energy into electrical energy through redox (reduction-oxidation) reactions. It consists of two electrodes (an anode and a cathode) immersed in an electrolyte solution. When a chemical reaction occurs, electrons flow from the anode to the cathode, generating an electric current.
Components of a Galvanic Battery
- Anode: The negative electrode where oxidation occurs. During this process, electrons are released.
- Cathode: The positive electrode where reduction takes place. Here, electrons are gained.
- Electrolyte: A conductive solution or gel that allows ions to move between the anode and cathode, completing the electrical circuit.
- Separator: A material that physically separates the anode and cathode to prevent short-circuiting while allowing ion flow.
How Do Galvanic Batteries Work?
The functioning of a galvanic battery can be summarized in a few key steps:
- Chemical Reaction: At the anode, a chemical reaction occurs that releases electrons. For example, in a zinc-copper battery, zinc oxidizes, releasing electrons.
- Electron Flow: The released electrons flow through an external circuit to the cathode, creating an electric current that can power devices.
- Ion Movement: Simultaneously, positive ions generated at the anode move through the electrolyte towards the cathode, maintaining charge balance.
- Reduction at the Cathode: At the cathode, a reduction reaction occurs as electrons combine with ions from the electrolyte.
Types of Galvanic Batteries
There are various types of galvanic batteries, each with unique characteristics:
- Primary Batteries: These are non-rechargeable batteries, such as alkaline batteries, commonly used in household items like remote controls and flashlights.
- Secondary Batteries: Rechargeable batteries, such as lithium-ion and nickel-metal hydride (NiMH) batteries, are used in smartphones, laptops, and electric vehicles.
- Fuel Cells: A type of galvanic cell that generates electricity through the reaction of hydrogen and oxygen, commonly used in some types of electric vehicles and stationary power applications.
Applications of Galvanic Batteries
Galvanic batteries play a crucial role in various applications:
1. Consumer Electronics
From smartphones and laptops to digital cameras, galvanic batteries provide the necessary power to keep our devices operational. Lithium-ion batteries, in particular, are favored for their high energy density and rechargeability.
2. Electric Vehicles (EVs)
As the demand for sustainable transportation grows, galvanic batteries are at the forefront of the electric vehicle revolution. They store energy efficiently, allowing for longer ranges and faster charging times. The development of solid-state batteries promises even greater advancements in this field.
3. Renewable Energy Storage
Galvanic batteries are essential for storing energy generated from renewable sources like solar and wind. They allow excess energy to be stored and used when production is low, enhancing the reliability of renewable energy systems.
4. Medical Devices
In the medical field, galvanic batteries power critical devices such as pacemakers and portable diagnostic equipment. Their reliability and longevity are crucial for patient care.
5. Military and Aerospace
Galvanic batteries are used in various military applications, including drones and communication devices. Their lightweight and durable nature makes them ideal for use in harsh environments.
Conclusion
Understanding galvanic batteries is crucial as we navigate an increasingly electrified world. Their ability to convert chemical energy into electrical energy has made them indispensable in a wide range of applications, from everyday consumer products to advanced technologies. As research and development continue to advance, the future of galvanic batteries promises even more innovations, paving the way for a sustainable and electrified future.
In summary, galvanic batteries are not just a technological necessity; they are a fundamental part of the global shift towards renewable energy and sustainable practices. Understanding their workings and applications helps us appreciate their role in powering our lives today and in the future.